17016-83-0 (S)-(-)-4-Isopropyl-2-oxazolidinone
-
 * For R&D use only.In Production Stage,Request Bulk Quotation
* For R&D use only.In Production Stage,Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| Accela | SY007944 | 1g | 15.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
| Accela | SY007944 | 5g | 15.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY007944 | 10g | 17.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
| Accela | SY007944 | 25g | 25.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY007944 | 100g | 99.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
Alkyl carboxamides derived from the N-acylation of the oxazolidinone form enolates which undergo highly enantioselective aldol condensation with aldehydes. The products readily undergo hydrolysis, e.g. with base, to give chiral ? -hydroxyacids, with consequent easy recovery and recycle of the chiral auxiliary: J. Am. Chem. Soc., 103, 2127 (1981); Tetrahedron, 27, 897 (1986); Tetrahedron Lett., 31, 4699 (1990); J. Org. Chem., 56, 2489 (1991). The enolates of the N-acyloxazolidinones can also undergo a variety of other enantioselective reactions including alkylation: J. Am. Chem. Soc., 104, 1737 (1982); acylation: J. Am. Chem. Soc., 106, 1154 (1984). Bromination (NBS), followed by azide displacement, gives a route to chiral amino acids: Tetrahedron Lett ., 28, 1123 (1987). Direct electrophilic azidation with triisopropylbenzenesulfonyl azide has also been used to effect this transformation: J. Am. Chem. Soc., 112, 4011 (1990).
Related Products
 SY023072
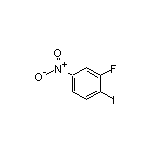
SY023072
CAS:2996-30-7
3-Fluoro-4-iodonitrobenzene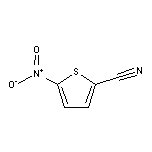 SY011987
SY011987
CAS:16689-02-4
5-Nitro-2-thiophenecarbonitrile SY012592
SY012592
CAS:68547-97-7
1,2,3,6-Tetrahydro-4-pyridinecarboxylic Acid Hydrochloride![5-Chlorobenzo[b]thiophene](/shaoyhuaxue/structure/SY021670.gif) SY021670
SY021670
CAS:20532-33-6
5-Chlorobenzo[b]thiophene SY022663
SY022663
CAS:33567-59-8
2-(2-Methoxyphenyl)acetaldehyde SY022899
SY022899
CAS:121602-93-5
3,4,5-Trifluorobenzoic Acid SY022012
SY022012
CAS:704-14-3
5-Methoxy-2-nitrophenol SY022109
SY022109
CAS:481704-21-6
(S)-4-Hydroxy-D-proline Methyl Ester Hydrochloride

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】








 沪公网安备 31011502016088号
沪公网安备 31011502016088号