516-12-1 N-Iodosuccinimide (NIS)
-
 * For R&D use only.In Production Stage,Request Bulk Quotation
* For R&D use only.In Production Stage,Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| Accela | SY001605 | 5g | 15.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
| Accela | SY001605 | 10g | 15.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY001605 | 25g | 15.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY001605 | 100g | 30.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY001605 | 500g | 116.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
Source of positive iodine. Iodinates methoxy benzenes and naphthalenes in acetonitrile, e.g. anisole gives 95% yield of 4-iodoanisole: Tetrahedron Lett., 37, 4081 (1996). In combination with TFA and TFA anhydride, iodinates 2,4-diethoxypyrimidines or N-alkyluracils specifically to their 5-iodo-derivatives: Synth. Commun., 18, 855 (1988). With triflic acid, the "superelectrophile" iodine(I) triflate is formed. This species will iodinate even deactivated aromatics, e.g. nitrobenzene to the m-iodo derivative: J. Org. Chem., 58, 3194 (1993). Alone or with a catalytic amount of triflic acid, is a powerful coupling agent in oligosaccharide synthesis, particularly for thioglycosyl donors; see, e.g.: Tetrahedron Lett., 34, 8523 (1993). For reviews, see: Chem. Rev., 93, 1503 (1993); Contemp. Org. Synth., 3, 173 (1996).
Related Products
 SY001614
SY001614
CAS:1076-38-6
4-Hydroxycoumarin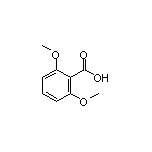 SY001616
SY001616
CAS:1466-76-8
2,6-Dimethoxybenzoic Acid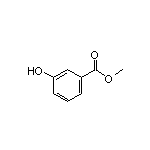 SY001617
SY001617
CAS:19438-10-9
Methyl 3-Hydroxybenzoate SY001624
SY001624
CAS:455-24-3
4-(Trifluoromethyl)benzoic Acid SY001629
SY001629
CAS:2009-83-8
6-Chloro-1-hexanol SY001630
SY001630
CAS:107-97-1
Sarcosine SY001649
SY001649
CAS:3034-53-5
2-Bromothiazole SY001650
SY001650
CAS:103-16-2
4-Benzyloxyphenol

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】








 沪公网安备 31011502016088号
沪公网安备 31011502016088号