14694-95-2 Tris(triphenylphosphine)rhodium(I) Chloride
-
 * For R&D use only.Request Bulk Quotation
* For R&D use only.Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| Accela | SY007812 | 25g | 1331.00 | 0 | 0 | inquire | Login | ||||||||||||
| Accela | SY007812 | bulk-g | POA | - | - | inquire | Login | ||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
Homogeneous hydrogenation catalyst: J. Chem. Soc.(A), 1711 (1966), useful e.g. for the selective reduction of an unhindered alkene, of an unconjugated in the presence of a conjugated alkene: Org. Synth. Coll., 6, 459 (1988), or an alkene in the presence of a nitro group: J. Org. Chem., 67, 3163 (2002). Hydroxyl groups protected as their allyl ethers may be deprotected by isomerization with Wilkinson's Catalyst to the more readily-hydrolyzed 1-propenyl ether: J. Org. Chem., 38, 3224 (1973). Aldehydes undergo decarbonylation with the complex: Tetrahedron Lett., 3969 (1965); J. Am. Chem. Soc., 93, 5465 (1971). The need for stoichiometric amounts of the complex, due to formation of an inactive Rh carbonyl complex, is a serious disadvantage. However, in the presence of Diphenyl-phosphonic azide, which regenerates the catalyst from the carbonyl complex, decarbonylations can be carried out catalytically at room temperature, providing a much more cost-effective and attractive method for this type of transformation: J. Org. Chem., 57, 5075 (1992). Catalyst for hydrosilylation reactions, e.g. with Triethyl-silane, including ɑ?-unsaturated ketones to silyl enol ethers, which can be hydrolyzed to saturated ketones: Organometallics, 1, 1390 (1982), and ɑ?-unsaturated esters to silyl ketene acetals with high (Z)-selectivity: Synth. Commun., 17, 1 (1989). Used by Grigg for the catalytic [2+2+2] cyclotrimerization of alkynes, providing an efficient route to benzene-fused ring systems. See, e.g.: J. Chem. Soc., Perkin 1, 1357 (1988). For an intermolecular example with reaction scheme, see 1,6-Heptadiyne. Intramolecular assembly of suitably constructed triynes can also be accomplished to form benzene rings: Tetrahedron, 45, 6239 (1989). Also catalyzes the [5+2] cycloaddition of vinylcyclopropanes and alkynes: J. Am. Chem. Soc., 117, 4720 (1995); 120, 1940 (1998). Co-catalyst giving improved results in intramolecular Heck coupling reactions catalyzed by Pd(OAc)2: J. Org. Chem., 64, 3461 (1999). Electron-deficient olefins undergo Rh-catalyzed 1,4-addition with Bis(pinacolato)-diboron, e.g. 2-cyclohexen-1-one to the ?-borylcyclohexanone: Tetrahedron Lett., 43, 2323 (2002).
Related Products
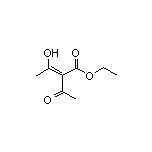 SY007998
SY007998
CAS:1830-94-0
Ethyl 2-Acetyl-3-hydroxy-2-butenoate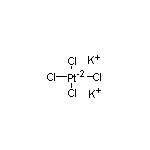 SY011802
SY011802
CAS:10025-99-7
Potassium Tetrachloroplatinate(II) SY001214
SY001214
CAS:130290-79-8
4-Aminomethyltetrahydropyran SY001994
SY001994
CAS:122536-76-9
(S)-3-(Boc-Amino)pyrrolidine SY002845
SY002845
CAS:2199-44-2
Ethyl 3,5-Dimethylpyrrole-2-carboxylate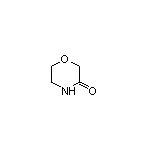 SY003974
SY003974
CAS:109-11-5
3-Morpholinone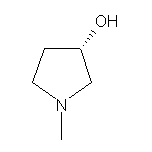 SY003953
SY003953
CAS:104641-59-0
(S)-(+)-1-Methyl-3-pyrrolidinol SY003828
SY003828
CAS:2026-48-4
L-(+)-Valinol

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】








 沪公网安备 31011502016088号
沪公网安备 31011502016088号