1774-47-6 Trimethylsulfoxonium Iodide
-
 * For R&D use only.In Production Stage,Request Bulk Quotation
* For R&D use only.In Production Stage,Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| Accela | SY002188 | 25g | 15.00 | in stock | 0 | 1 day | Login | ||||||||||||
| Accela | SY002188 | 100g | 20.00 | in stock | 0 | 1 day | Login | ||||||||||||
| Accela | SY002188 | 500g | 75.00 | 0 | 0 | 1week | Login | ||||||||||||
| Accela | SY002188 | bulk-g | POA | - | - | 1week | Login | ||||||||||||
| Accela | SY002188 | 1000g | 140.00 | 0 | 0 | 1week | Login | ||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
For an example (conversion of cyclohexanone to the epoxide), see: Org. Synth. Coll., 5, 755 (1973). In contrast to Trimethyl-sulfonium iodide, reaction with ɑ?-unsaturated ketones occurs by 1,4-addition to give cyclopropanes: J. Am. Chem. Soc., 87, 1353 (1965). Efficient addition to enones has been effected with a highly basic system consisting of KOH in DMSO with a phase-transfer catalyst: Synth. Commun., 26, 1785 (1996). Reaction with epoxides gives oxetanes, unlike the sulfonium ylide which gives allylic alcohols: J. Org. Chem., 48, 5133 (1983); Synthesis, 1140 (1987); cf Tetrahedron Lett., 35, 5449 (1994).Similarly, N-arenesulfonylaziridines undergo ring expansion to azetidines with inversion at one carbon. Thus, cis-substituted aziridines give trans-azetidines and vice versa: Tetrahedron, 45, 1851 (1989).
Related Products
 SY002226
SY002226
CAS:623-33-6
Glycine Ethyl Ester Hydrochloride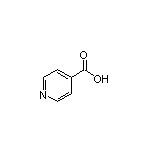 SY002246
SY002246
CAS:55-22-1
Isonicotinic Acid SY002250
SY002250
CAS:17455-13-9
1,4,7,10,13,16-Hexaoxacyclooctadecane (18-Crown-6)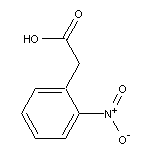 SY002275
SY002275
CAS:3740-52-1
2-Nitrophenylacetic Acid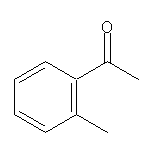 SY002283
SY002283
CAS:577-16-2
2’-Methylacetophenone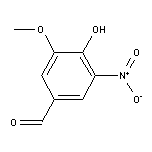 SY002288
SY002288
CAS:6635-20-7
5-Nitrovanillin SY002291
SY002291
CAS:1492-24-6
(S)-2-Aminobutanoic Acid SY002325
SY002325
CAS:586-38-9
3-Methoxybenzoic Acid

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】








 沪公网安备 31011502016088号
沪公网安备 31011502016088号