51364-51-3 Tris(dibenzylideneacetone)dipalladium(0)
-
 * For R&D use only.In Production Stage,Request Bulk Quotation
* For R&D use only.In Production Stage,Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| No match found. | |||||||||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
For use in the coupling of aryl- or vinyltin reagents with allyl halides, see: J. Am. Chem. Soc., 105, 7173 (1983). For Suzuki coupling of boronic acids with carbapenem triflates, see: Tetrahedron Lett., 34, 3211 (1993). Catalyzes the reduction of terminal allylic acetates or carbonates to 1-alkenes, with virtually complete regioselectivity: Synthesis, 623 (1986). In the presence of allyl bromide, catalyzes the coupling of terminal alkynes to symmetrical diynes under phase-transfer conditions. The reaction is thought to involve a π-allyl Pd intermediate: Tetrahedron, 52, 1337 (1996). In the presence of a chelating phosphine ligand and NaO-t-Bu, bromopyridines can be aminated: J. Org. Chem., 61, 7240 (1996).Catalyst for Stille and Heck coupling reactions in supercritical CO2, in combination with, e.g. Tris[3,5-bis(trifluoromethyl)-phenyl-]phosphine: Chem. Commun., 1397 (1998). Catalyzes the hydroxycarbonylation of aryl and vinyl halides or triflates by lithium formate, to give carboxylic acids: Org. Lett., 5, 4269 (2003).
Related Products
 SY002008
SY002008
CAS:12150-46-8
1,1'-Bis(diphenylphosphino)ferrocene (DPPF) SY002264
SY002264
CAS:15629-92-2
1,3-Bis(diphenylphosphino)propane Nickel (II) Chloride SY002294
SY002294
CAS:114615-82-6
Tetrapropylammonium Perruthenate (TPAP)![[1,1'-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) Complex With Dichloromethane](/shaoyhuaxue/structure/SY002614.gif) SY002614
SY002614
CAS:95464-05-4
[1,1'-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) Complex With Dichloromethane SY003233
SY003233
CAS:50982-12-2
Dichloro(1,5-cyclooctadiene)ruthenium(II) SY007812
SY007812
CAS:14694-95-2
Tris(triphenylphosphine)rhodium(I) Chloride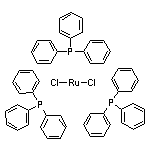 SY009924
SY009924
CAS:15529-49-4
Tris(triphenylphosphine)ruthenium(II) Dichloride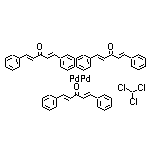 SY010270
SY010270
CAS:52522-40-4
Tris(dibenzylideneacetone)dipalladium(0)-chloroform Adduct

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】








 沪公网安备 31011502016088号
沪公网安备 31011502016088号