40615-36-9 4,4’-Dimethoxytrityl Chloride
-
 * For R&D use only.In Production Stage,Request Bulk Quotation
* For R&D use only.In Production Stage,Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| Accela | SY001416 | 5g | 15.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
| Accela | SY001416 | 10g | 15.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY001416 | 25g | 15.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY001416 | 100g | 30.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY001416 | 500g | 75.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
Reagent for protection of alcohols, compare Chlorotriphenyl-methane, as their dimethoxytrityl (Dmt) ethers. Mono-, di- and trimethoxytrityl chlorides form ethers of increasing acid-lability, each MeO group increasing the rate of cleavage by ca x10: J. Am. Chem. Soc., 84, 430 (1962); 85, 3821 (1963). The group is introduced e.g. by reaction with the alcohol in pyridine solution. For use of DMAP as catalyst, see: J. Am. Chem. Soc., 104, 1316 (1982), or of silver nitrate: Can. J. Chem., 60, 1106 (1982). For selective protection and deprotection procedures for SH and OH groups in nucleoside derivatives, see: Synlett, 83 (1993). In a comparison with Mmt for N-protection during solid-phase peptide synthesis, the N-Mmt group was found to survive intact whereas N-Dmt derivatives decomposed slowly in protic solvents: Tetrahedron. Lett., 39, 1733 (1998).
Related Products
 SY001428
SY001428
CAS:56-09-7
2-Amino-4,6-dihydroxypyrimidine SY001430
SY001430
CAS:539-74-2
Ethyl 3-Bromopropanoate SY001440
SY001440
CAS:1761-61-1
5-Bromosalicylaldehyde SY001445
SY001445
CAS:91-16-7
1,2-Dimethoxybenzene SY001447
SY001447
CAS:96-50-4
2-Aminothiazole SY001450
SY001450
CAS:121-34-6
Vanillic Acid SY001454
SY001454
CAS:13139-17-8
N-(Benzyloxycarbonyloxy)succinimide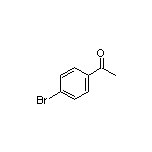 SY001458
SY001458
CAS:99-90-1
4’-Bromoacetophenone

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】








 沪公网安备 31011502016088号
沪公网安备 31011502016088号