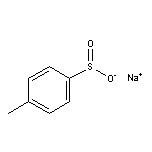
824-79-3 Sodium p-Toluenesulfinate
-
 * For R&D use only.In Production Stage,Request Bulk Quotation
* For R&D use only.In Production Stage,Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| Accela | SY013741 | 25g | 15.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY013741 | 100g | 34.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY013741 | 500g | 75.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
| Accela | SY013741 | bulk-g | POA | 0 | in stock | 3-5 days | Login | ||||||||||||
| Accela | SY013741 | 1000g | 125.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
Alkylation with reactive halides leads to alkyl sulfones. Alkylation of sulfinates can be carried out in a polyethylene glycol as solvent/catalyst: Bull. Chem. Soc. Jpn., 57, 613 (1984). Reaction with allylic alcohols also gives sulfones: J. Org. Chem., 55, 3274 (1990). For asymmetric sulfonylation of allylic chlorides, catalyzed by a chiral Pd complex, see: Tetrahedron: Asymmetry, 6, 643 (1995). For use as a precursor of p-toluenesulfonyldiazomethane, via Mannich reaction with formaldehyde and urethane, nitrosation and cleavage with alumina, see: Org. Synth. Coll., 6, 981 (1988). Also undergoes free-radical reactions in the presence of Cu(II), activated either thermally or by sonication. For example, addition-cyclization of 5-heteroarylpent-1-enes: Synlett, 763 (1995).
Related Products
 SY013750
SY013750
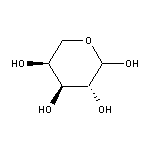
CAS:87-72-9
L-(+)-Arabinose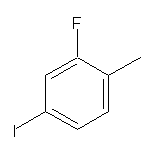 SY013795
SY013795
CAS:39998-81-7
2-Fluoro-4-iodotoluene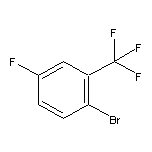 SY013796
SY013796
CAS:40161-55-5
2-Bromo-5-fluorobenzotrifluoride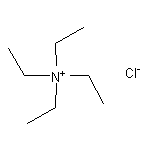 SY013931
SY013931
CAS:56-34-8
Tetraethylammonium Chloride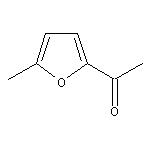 SY013987
SY013987
CAS:1193-79-9
2-Acetyl-5-methylfuran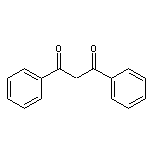 SY014045
SY014045
CAS:120-46-7
Dibenzoylmethane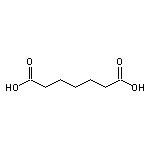 SY014051
SY014051
CAS:111-16-0
Pimelic Acid SY014104
SY014104
CAS:99-50-3
3,4-Dihydroxybenzoic Acid

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】








 沪公网安备 31011502016088号
沪公网安备 31011502016088号