108448-77-7 (1R)-(+)-2,10-Camphorsultam
-
 * For R&D use only.In Production Stage,Request Bulk Quotation
* For R&D use only.In Production Stage,Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| Accela | SY004517 | 1g | 15.00 | 0 | in stock | 3-5 days | Login | ||||||||||||
| Accela | SY004517 | 5g | 15.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY004517 | 10g | 20.00 | in stock | in stock | 1 day | Login | ||||||||||||
| Accela | SY004517 | 25g | 27.00 | in stock | 0 | 1 day | Login | ||||||||||||
| Accela | SY004517 | 100g | 107.00 | 0 | 0 | inquire | Login | ||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
Versatile chiral auxiliary introduced by Oppolzer. Review: Tetrahedron, 49, 293 (1993). N-Acryloyl derivatives undergo enantioselective cycloaddition reactions: Helv. Chim. Acta, 67, 1397 (1984); Tetrahedron, 43, 1969 (1987). Hydrogenation of N-acryloyl derivatives proceeds with high diastereoselectivity: Tetrahedron Lett., 27, 183 (1986); Helv. Chim. Acta, 69, 1542 (1986), 69, 1817 (1986); 70, 1666 (1987). Induces asymmetric conjugate addition of Grignard reagents to N-acryloyl derivatives: Helv. Chim. Acta, 70, 2201 (1987). In the presence of strong bases, N-acyl derivatives undergo stereoselective alkylation: Tetrahedron Lett., 30, 5603 (1989); or aldol-type condensation reactions with aldehydes: J. Am. Chem. Soc., 112, 2767 (1990); Tetrahedron Lett., 32, 61 (1991). Chiral auxiliary for the asymmetric Baylis-Hillman reaction: J. Am. Chem. Soc., 119, 4317 (1997).
Related Products
 SY004518
SY004518
CAS:94594-90-8
(1S)-(-)-2,10-Camphorsultam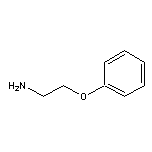 SY004523
SY004523
CAS:1758-46-9
2-Phenoxyethylamine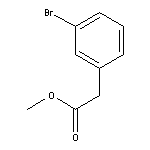 SY004524
SY004524
CAS:150529-73-0
Methyl 2-(3-Bromophenyl)acetate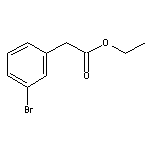 SY004525
SY004525
CAS:14062-30-7
Ethyl 3-Bromophenylacetate SY004526
SY004526
CAS:2923-56-0
4-(Trifluoromethyl)phenylhydrazine Hydrochloride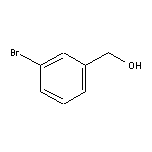 SY004527
SY004527
CAS:15852-73-0
3-Bromobenzyl Alcohol SY004529
SY004529
CAS:1679-18-1
4-Chlorobenzeneboronic Acid SY004531
SY004531
CAS:91076-93-6
Methyl 3-Amino-5-(4-chlorophenyl)thiophene-2-carboxylate

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】








 沪公网安备 31011502016088号
沪公网安备 31011502016088号