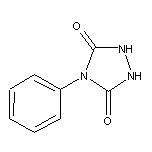
15988-11-1 4-Phenylurazole
-
 * For R&D use only.Request Bulk Quotation
* For R&D use only.Request Bulk Quotation
| Brand | CatalogID | Unit | Price($) | Stock (US) | Stock (CN) | Ships within | Quantity | Buy | |||||||||||
| No match found. | |||||||||||||||||||
Please Login to see Special Offer & Quantity. | |||||||||||||||||||
Product InformationSafety InformationReferences
| Flash point | |
| Boiling point | |
| Density | |
| Storage |
Oxidation gives the aza-dienophile 4-phenyl-1,2,4-triazolinedione which is more reactive in cycloaddition reactions than tetracyanoethylene: J. Chem. Soc. (C), 1905 (1967); Angew. Chem. Int. Ed., 11, 715 (1972); Org. Prep. Proced. Int., 7, 251 (1975). Suitable oxidants include t-butyl hypochlorite: Org. Synth. Coll., 6, 936 (1988), or lead(IV) acetate: J. Org. Chem., 32, 330 (1967). The adducts can be cleaved to the hydrazo-compounds by hydrazine hydrate. Cu(II) oxidation provides a route to azoalkanes: Synthesis, 543 (1981). Reacts with enolizable ketones in the presence of TFA to give ɑ-urazyl ketones: J. Org. Chem., 55, 193 (1990), which may be oxidized to ɑ-diketones with t-butyl hypochlorite: J. Org. Chem., 55, 197 (1990). Review of use of azodicarbonyl compounds in synthesis: Adv. Het. Chem., 30, 1 (1982). 1,2,4-Triazolinediones form charge-transfer complexes with electron-rich aromatics, such as di- or trimethoxybenzenes: J. Org. Chem., 48, 1708 (1983).
Related Products
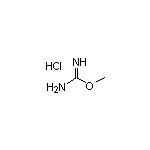 SY023512
SY023512
CAS:5329-33-9
O-Methylisourea Hydrochloride![1-[3-(Dimethylamino)propyl]-3-ethylurea](/shaoyhuaxue/structure/SY027122.gif) SY027122
SY027122
CAS:32897-26-0
1-[3-(Dimethylamino)propyl]-3-ethylurea![3-[4’-(Dimethylamino)-biphenyl-3-yl]-1,1-dimethylurea](/shaoyhuaxue/structure/SY038696.gif) SY038696
SY038696
CAS:1469924-27-3
3-[4’-(Dimethylamino)-biphenyl-3-yl]-1,1-dimethylurea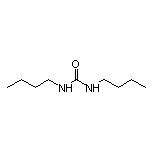 SY037877
SY037877
CAS:1792-17-2
N,N’-Dibutylurea SY004507
SY004507
CAS:96-31-1
N,N’-Dimethylurea SY038266
SY038266
CAS:656-32-6
1-(2-Fluorophenyl)thiourea SY021749
SY021749
CAS:52328-05-9
O-Methylisourea Hemisulfate Salt SY018863
SY018863
CAS:598-50-5
N-Methylurea

 (858)699-3322
(858)699-3322
 【COA&SDS】
【COA&SDS】







 沪公网安备 31011502016088号
沪公网安备 31011502016088号